Laser light is generated in the active medium of the laser. Energy is pumped into the active medium in an appropriate form and is partially transformed into radiation energy. The energy pumped into the active medium is usually highly entropic, i.e. very disorganised, while the resulting laser radiation is highly ordered and thus has lower entropy. Highly entropic energy is therefore converted into less entropic energy within the laser. Active laser media are available in all aggregate states:
- solid (crystalline or amorphous)
- liquid
- gaseous or as plasma
The way in which the energy is introduced is highly dependent on the aggregate state. Many gaseous laser media can, for instance, be stimulated with the aid of gas discharge. This facilitates the transfer of electrical energy to free electrons which, in turn, emit their energy on collision with atoms or molecules. Solid state and liquid lasers can onlybe pumped optically; radiation from an ordinary lamp or another laser is absorbed by the active medium and the energy is re-emitted at longer wavelengths.
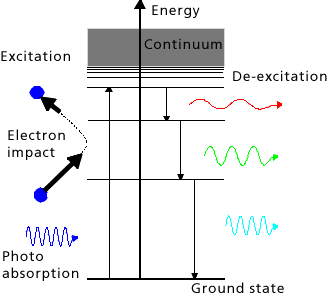
Atoms and molecules are normally present in the so-called ground state. This is a stable condition - atoms in the ground state cannot release energy. Atoms can exist in other states inwhich there is more energy present than in the ground state - a supply of energy is needed to raise the atoms to these higher states. The energy can be provided by other particles, particularly free electrons, or light quanta (photons). The atoms can return to lower energy levels (e.g. to the stable ground state) from this ‘excited’ state by releasing energy. The surplus energy can be passed on to another particle, e.g. an electron or another atom, or a photon which is then emitted - the energy of the photon is equivalent to the difference in energy between the higher and lower states. This de-excitation can be spontaneous or stimulated by other photons. Stimulated emission is a basic requirement for lasing.
 Fraunhofer Institute for Laser Technology ILT
Fraunhofer Institute for Laser Technology ILT